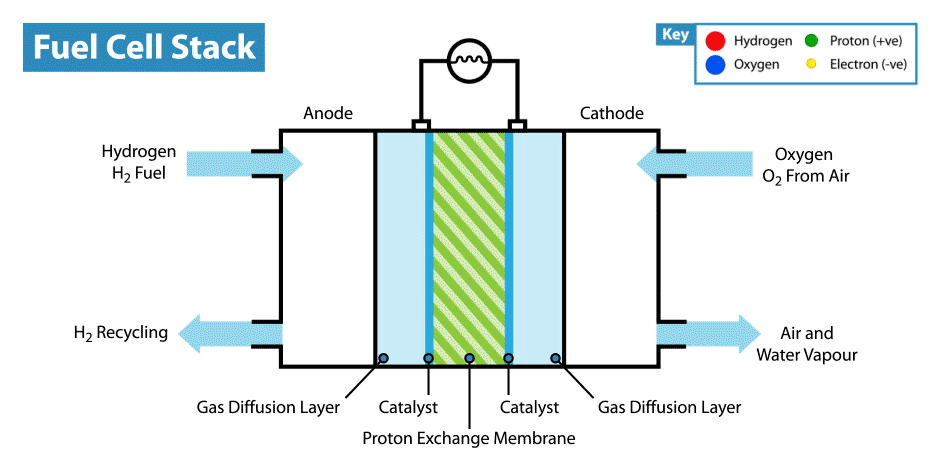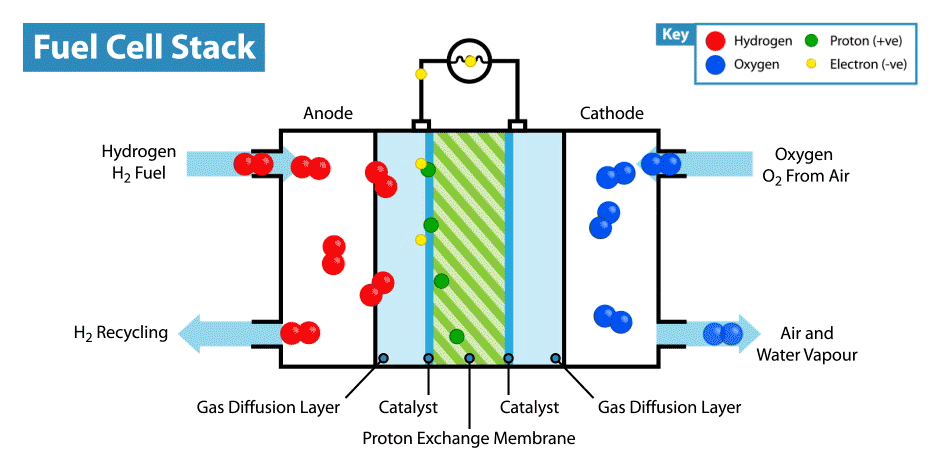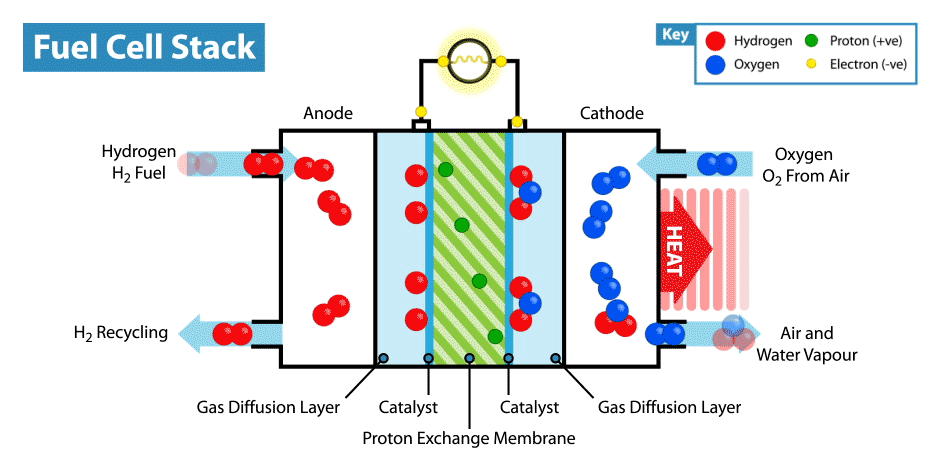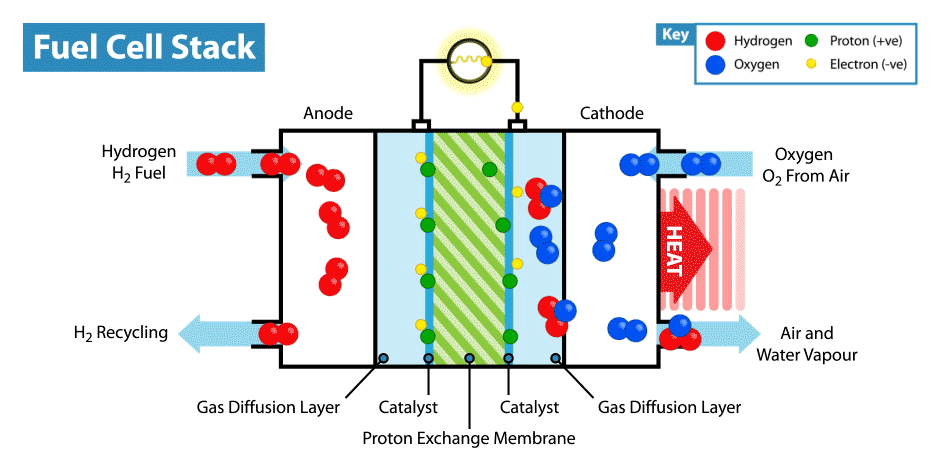
A fuel cell is an electrochemical device that produces electricity without combustion. The optimal energy carrier for fuel cells is hydrogen (which can be extracted for example from water, methanol, natural gas or petroleum products). When hydrogen is combined with oxygen (from air) it produces electrical energy. The conversion process is environmentally benign: only heat and water are emitted as by-products. Environmentally, hydrogen is the optimal energy carrier for fuel cells, because fuel cells that run on hydrogen have zero emissions.
By the nature of its electrochemical reaction, a fuel cell can be more than twice as efficient as an internal combustion engine. A conventional engine burns fuel to create heat and in turn converts heat into mechanical energy and finally electricity. A fuel cell produces electricity, water and heat directly from hydrogen and oxygen. Fuel cells are like batteries in that they are electrochemical devices, but unlike batteries do not need recharging and will continue to operate as long as they are provided with fuel (hydrogen).